Notification No.: 56/2025-26
Date: 29 January 2026
Issued by: Directorate General of Foreign Trade (DGFT)
Effective: Immediately (valid for one year)
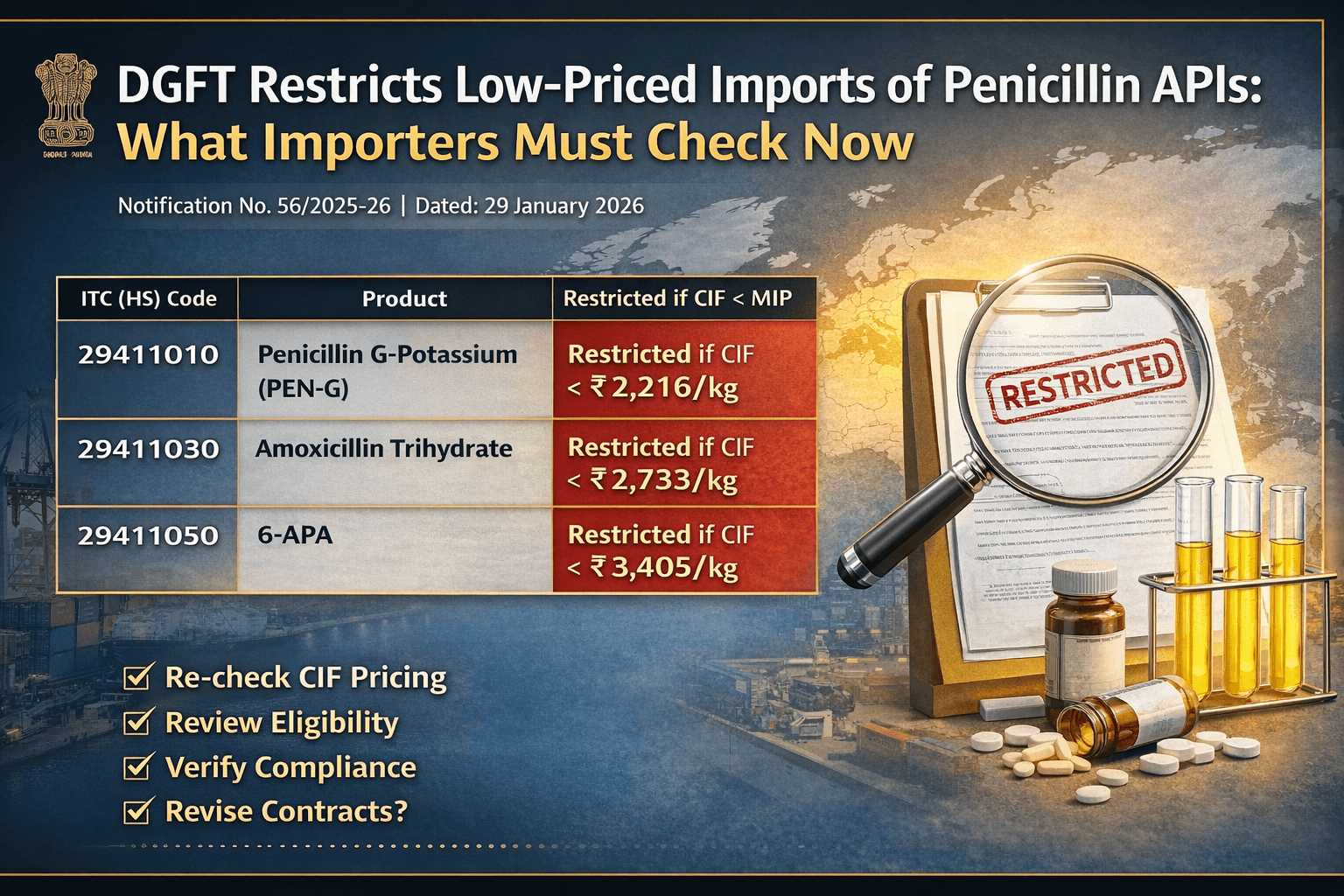
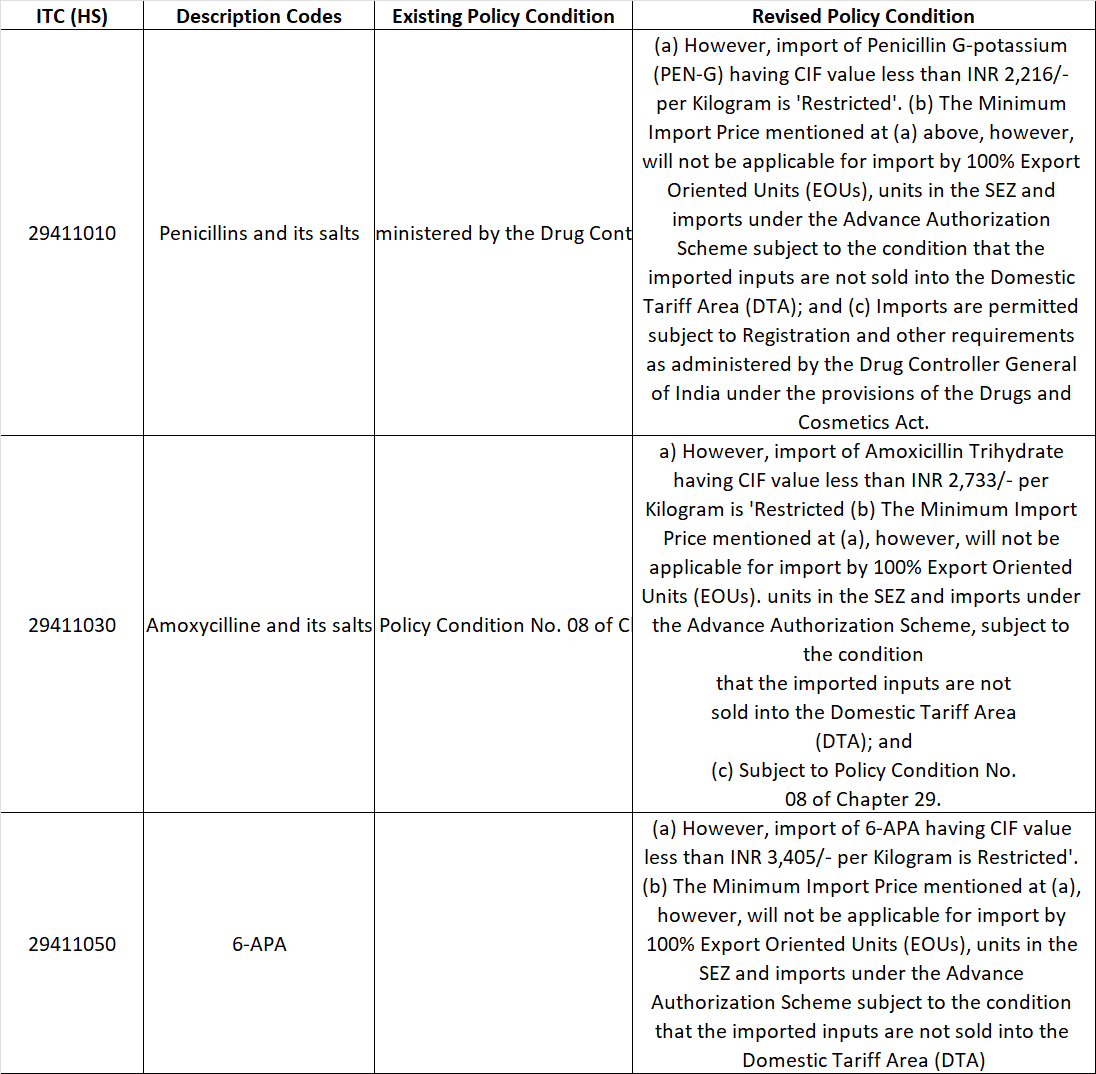
The Directorate General of Foreign Trade (DGFT) has issued Notification No. 56/2025-26, amending the import policy and policy conditions for certain Penicillin-based pharmaceutical inputs under Chapter 29 of ITC (HS) 2022, Schedule-I (Import Policy).
The amendment introduces Minimum Import Price -MIP - linked restrictions while keeping the overall import policy as “Free”, subject to specified conditions.
What has changed -
Although the import policy remains “Free”, DGFT has effectively restricted low-priced imports by introducing Minimum Import Prices (MIP).
If CIF value is below the MIP:
Import becomes Restricted
DGFT authorisation is required
Clearance may be denied without proper approval
Who Is exempted from MIP restrictions?
The following categories are exempt from the Minimum Import Price restriction, provided strict conditions are met:
100% Export Oriented Units (EOUs)
Units located in Special Economic Zones (SEZs)
Imports under Advance authorisation Scheme
This move is widely seen to:
Protecting domestic API manufacturers
Preventing dumping of under-priced pharmaceutical inputs
Strengthening India’s pharma supply chain resilience
Ensuring quality and regulatory oversight in sensitive drug imports
What Importers should do now
Review CIF pricing in existing and future contracts
Re-check HS code classification carefully
Ensure eligibility if importing under EOU / SEZ / Advance Authorisation
Maintain strict DTA-non-sale compliance records
Consult DGFT / Customs experts before filing Bills of Entry below MIP thresholds
Popular Posts
Explore Topics
Comments